Stoichiometry
A. STOICIOMETRY DEFENITION
Stoichiometry is the study of the quantitative relationships or ratios between two or more substances undergoing a physical change or chemical change (chemical reaction).
Stoichiometry is the calculation of relative quantities of reactan and product in chemical reaction
B. Factors to Stoichiometry
- Balance the equation.
- Convert units of a given substance to moles.
- Using the mole ratio, calculate the moles of substance yielded by the reaction.
- Convert moles of wanted substance to desired units.
C. Formula
1.The gram formula mass can be used as a conversion factor in stoichiometric calculations through the following equation:
Moles = 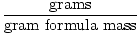 |
2.Volume of a Gas and Moles
| n = |
with P representing pressure in atm, Vrepresenting volume in liters, Trepresenting temperature in Kelvins, and R the gas constant, which equals .0821 L-atm/mol-K. Given P , V , and T , you can calculate the number of moles of substance in a gas.
3. Some representative formula units are listed below.
- Compounds: Cu2S , NaCl
- Molecules: N2 , H2
- Atoms: Fe, Na
- Ions: Na+(aq) , Cl-(aq)
Moles = 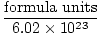 |
4.molarity and molality
Molarity is defined as the number of moles of solute divided by the number of liters of solvent. Rearranging the equation to solve for moles yields:
Moles = molarity × liters of solution
MolaLity is defined as the number of moles of solute divided by the number of kilograms of solvent. Rearranging the equation to solve for moles yields:
Moles = molality × kilograms of solution
This my vidio : explain about stoiciometry.


please explain about how to use stoichiometry in chemical calculations? thanks
BalasHapusThe subject of stoichiometry involves quantitative calculations based on chemical formulas and chemical equations.
Hapus3.1 Molecular Masses and Formula Masses—Molecular masses and formula masses are the masses, expressed in atomic mass units (u), of individual molecules and formula units. They are calculated from the masses of the atoms represented in the molecular or empirical formulas, respectively. Molecular mass applies only to molecular compounds; formula mass is appropriate for ionic compounds.
3.2 The Mole and Avogadro’s Number—A mole (mol) is an amount of substance containing a number of elementary entities (atoms, molecules, formula units, etc.) equal to the number of atoms in exactly 12 g of carbon-12. This number is Avogadro’s number, NA = 6.022 × 1023 mol-1. The mole is the SI unit for the amount of a substance and is used extensively in chemical equations and calculations.
What is the formula of stoichiometry?
BalasHapusI would give you example of stoiciometry ,because its formula very much
HapusThe reaction of ammonia with solid copper oxide give the nitrogen gas, solid copper and water vapor. Write this reaction and balancing the equation according to its stoichiometry?
Solution:
The described question is in the form of,
NH3 + CuO →→ Cu + H2O + N2
Balancing the above equation according to its stoichiometry,
2NH3 + 3CuO →→ 3Cu + 3H2O + N2
Please give formula to moles ?
BalasHapusMoles to grams formula: grams(g) = moles(n) X molecular mass
HapusAnd you can use this :
n = gr/mr
Explain about avogadro's law?
BalasHapusAvogadro's law (sometimes referred to as Avogadro's hypothesis or Avogadro's principle) is an experimental gas law relating volume of a gas to the amount of substance of gas present.[1] A modern statement of Avogadro's law is:
HapusAvogadro's law states that, "equal volumes of all gases, at the same temperature and pressure, have the same number of molecules"
What disinguishes molality from molarity?
BalasHapusMolarity, also known as molar concentration, is the number of moles of a substance per liter of solution. Solutions labeled with the molar concentration are denoted with a capital M. A 1.0 M solution contains 1 mole of solute per liter of solution.
HapusMolality is the number of moles of solute per kilogram of solvent. It is important the mass of solvent is used and not the mass of the solution. Solutions labeled with molal concentration are denoted with a lower case m. A 1.0 m solution contains 1 mole of solute per kilogram of solvent.
why stoichiometry is very important in chemistry ?
BalasHapusThere are a number of reasons why chemistry students study stoichiometry. I'd say the most important is the ability to make useful predictions.
HapusExplanation:
Stoichiometry allows us to make predictions about the outcomes of chemical reactions. Making useful predictions is one of the main goals of science, the other being the ability to explain phenomena we observe in the natural world.
So what kind of predictions can we make using stoich? Here are some examples:
Predict the mass of a product of a chemical reaction if given the starting masses of reactants.
Predict the volume of a gas which will be produced by a reaction if given the starting amounts of reactants.
Determine the optimal ratio of reactants for a chemical reaction so that all reactants are fully used.
can you explain function of stoikiometry in the daily life?
BalasHapusWhat do cookies and chemistry have in common? Many things, it turns out! A balanced chemical equation is the recipe for a reaction: it contains a list of all the reactants (the ingredients) and products (the cookies) as well as their relative proportions.
Hapusgive me example please?
BalasHapusSulphur trioxide gas is obtained by the combustion of iron pyrites(FeS2). Write down the chemical reaction and balance this equation according to the stoichiometry of each compound?
HapusSolution:
According to the question, the equation for chemical reaction is
FeS2 + O2 →→ Fe2O3 + SO3
(While combustion compounds react with the oxygen)
Balancing the above equation according to stoichiometry is,
4FeS2 + 15O2 →→ 2Fe2O3 + 8SO3
What it is stoichiometry?
BalasHapusStoichiometry is the study of the quantitative relationships or ratios between two or more substances undergoing a physical change or chemical change (chemical reaction)
BalasHapusplease explain the stoichiometry implications on every life
BalasHapusThe principles of stoichiometry can be used while cooking.
BalasHapusIf you were almost out of a specific ingredient, you could use the principles of stoichiometry to figure out how much of every other ingredient you would need (treating the ingredient you were almost out of as the "limiting reagent").